
Drug clearance :
Once a drug enters into the body, the process of elimination begins. The three major routes of eliminations are:
(i) Hepatic metabolism
(ii) Biliary elimination
(iii) Urinary excreation
Excreation is irreversible removal of the drug from the body. It involves BIOTRANSFORMATION & EXCREATION.
•> Metabolism results in products with increase polarity, which allows the drug to be eliminated.
•> Clearance(CL) estimates the vomume of blood from which the drug is cleared per unit of time.
CL = 0.693 x Vd / t 1/2
- Where T 1/2 is the elimination half life, Vd is apparent volume of distribution and 0.693 is natural log.
BIOTRANSFORMATION
• The general principle of biotransformation is the metabolic conversion of drug mollecule to more water soluble metabolites that are more readily excreated.
- In many cases, metabolism of drug results in its conversion to compounds that have little or no pharmacological activity.
- In other cases, biotransformation of an active compound may lead to the formation of metabolites that also have pharamacologic actions.
- A few compounds (prodrugs) have no activity until they undergo metabolic ativation.
- Some compounds also converted into toxic metabolities.
Drug =====> Active metabolite
Drug =====> Inactive mtabolite
Prodrug =====> Drug
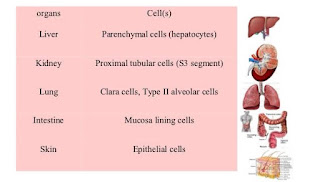
Sites of Biotransformation :
(i) LIVER : microsomal & non-microsomal enzyme system.
(ii) GIT : gastric acids, digestive enzymes, enzymes in wall of intestine & intestinal microorganisms.
(iii) Lungs
(iv) Kidney
(v) adrenal glands
(vi) Skin
Significance of Biotransformation :
• Termination of drug action.
• Activation of prodrug (eg : Clorazepate==>nordiapizam)
• Conversion of parent mollecule into an even more potent form.
Factors affecting biotranformation:
• Genetics : it may alter enzyme level or cause polymorphism of enzymes involved in Acetylation, Hydroxylation, & Hydrolysis.
• Route of administration : oral route can result in extensive first pass effect.
• Chemical properties of drugs : Certain drugs may stimulates or inhibit the metabolism of other drugs by inhibiting or activating P450 system in Liver.
• Dosage : Toxic dose can deplete enzyme, which decrease the biotranformation.
• Diet : starvation depletes glycine and can alter glycine conjugation.
• Age : In liver of newborn or neonates cannot detoxify drugs due to undeveloped drug metabolizing enzymes system in liver, conjugation reactions are not effective as well.
• Sex : young males are more prone to sedation from batbiturates than females.
• Disease : Liver disease decrease drug metabolism which cause increase drug action.
Phases of Biotranformation
• The kidney cannot effeciently excreates lipophilic drugs that readily cross cell membranes and are reabsorbed in DCTs. Therefore lipids soluble agents are first metabolized into more polar( Hydrophilic) substances in liver via two sets of reactions, called phase 1 and phase 2.
PHASE 1 :
• Also known as Enzymatic tranformation.
• It convert lipophilic drugs into more polar mollecules by introducing or unmasking a polar functional group, such as -OH or -NH2.
• Phase 1 reactions usually involve reduction, oxidation, or hydrolysis.
• Phase 1 metabolism may increase, decrease or no effect on pharmcological activity.
(1) OXIDATION :
• It involves the addition of O2 or release of H2 from drugs by microsomal or non-microsomal systems.
(i) Microsomal Metabolism :
• It cause drug oxidation by oxidative drug metabolizing enzymes, located in lipophilic membranes of smooth ER of liver and other tissue cells.
(a) Cytochrome P450 isozymes : major enzyme involved in phase 1 reactions located in smooth ER(microsomal fraction) of cells (especially liver but also GI tract, lungs , kidney) .
- P450 have an absolute requirement for mollecular oxygen and NADPH.
- Oxidations include hydroxylations and dealkylations.
- Multiple Cytochrome P families differing by Aminoacids composition, by substrate specificity, and by sensitive to inhibitors and to inducing agents.
(ii) Non-microsomal metabolism :
(a) Cytochrome P450 independent oxidation : it involves the oxidation of soluble enzymes found in cytosol or mitochondria of cell.
Example : monoamine oxidase, Alcohal dehydrognase, Aldehyde dehydrogenase, Xanthine oxidase, Tyrosine hydroxylase.
(b) Reduction : it occurs in both microsomal and non-mocrosomal metabolising system.
Example : azo-reduction , nitro-reduction etc.
(c) Hydroysis : It involves non-microsomal hydrolases present in various body systems including plasma.
- It involves addition of water mollecule with subsequent bond breakage.
Example : esterase , amidases.
(d) Alcohal metabolism : Alcohals are metabolised to aldehydes and then to acids by dehydrogenases.
Phase 2
• It involves coupling or conjugation reaction of parent drug or their Phase-1-metabolite with an endogenous substance to yeild drug conjugates.
• It is catalyzed by various transferases located in microsomes or in cytosol.
• Drug conjugates are polar mollecules that are readily excreated & often inactive.
(a) Glucuronidation :
• inducible , may undergo enterohepatic cycling.
( drug glucuronide => intestinal bacterial glucuronidase => free drug).
- Morphine is activated to more potent morphine-6-glucuronide.
- It is the most common and the most important conjugation reaction.
(b) Acetylation :
• Drug induce SLE by slow acetylators with hudralazine >procainamide> isoniazed(INH).
- Acetylation of sulfonamides.
(c) Glutathione (GSH) conjugation :
• Depletion of GSH in liver is associated with acetaminophen hepatotoxicity.
- Ethenacrynic acid
(d) Glycine conjugation : Salicylic acid.
(e) Methylation : Dopamine, Epinephrine, histamine.
(f) Water conjugation : Carbamazepine
• Drugs already possesing an -OH, -NH2, or -COOH group may enter phase 2 directly and become conjugated without prior phase 1 metabolism.
• The highly polar-drug-conjugates are then excreated by the kidney or in bile.





Comments
Post a Comment